© Compound InterestSourness & Scurvy – The Chemistry of a Lemon
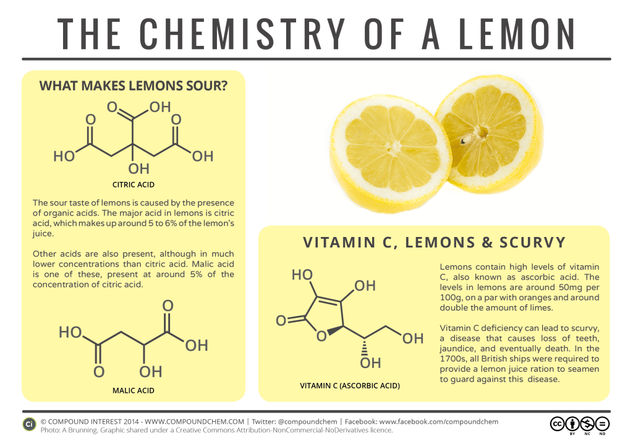
With it being Pancake Day tomorrow, it seemed like a good time to look at the chemistry of the humble lemon, and the compounds that give it its sour taste. Of course, citric acid is already well known – it even has its own E number (E330). However, a couple of other acid compounds are also contributors towards the chemical make up of a lemon.
One of these is malic acid, a compound that also has its own E number (E296). Citric acid is present in much higher quantities than malic acid and is the main contributor to the lemon’s sour taste; however, malic acid is present in around 5% of the concentration of citric acid. It is also found in apples and cherries, and responsible for aspects of their flavour.
Another acid present in lemons, and one with which citric acid is occasionally confused, is ascorbic acid, or vitamin C. The vitamin C levels in a lemon, at around 50 milligrams per 100 grams, are on a par with those of an orange, and significantly higher than those in a lime (~29mg/100g). This last fact in particular is one that the British Navy discovered belatedly to their detriment in the early 1900s.