http://www.compoundchem.com/2014/11/13/vsepr/VSEPR & Shapes of Molecules
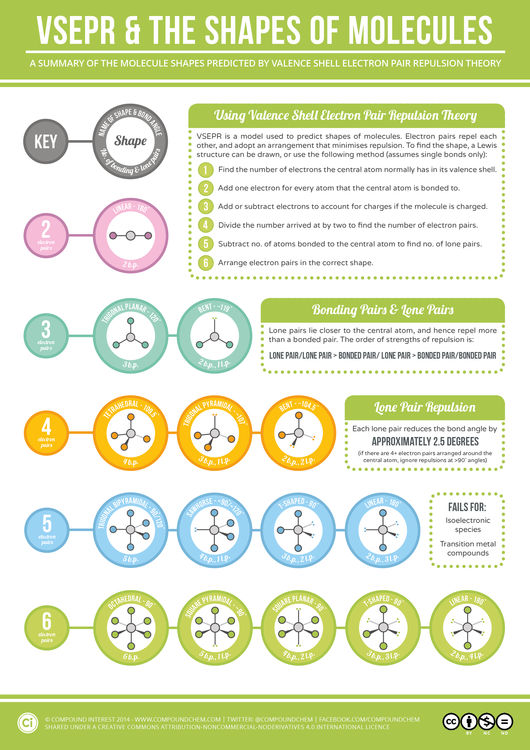
One for the chemistry students (and teachers!) out there today, with a look at how we can work out the shapes of some simple molecules using Valence Shell Electron Pair Repulsion (VSEPR) theory. These shapes are decided by the arrangement of electrons around the central atom in the molecule.
VSEPR works on the assumption that the shape adopted is that which minimises repulsions between the electron pairs in the molecule. It doesn’t take into account factors such as the size of bonded atoms or groups, and as such doesn’t always predict the shape of certain compounds correctly, in particular those of transition metals. However, it generally gives good predictions for compounds of main group elements.