© Compound InterestThe Chemistry of Fireworks
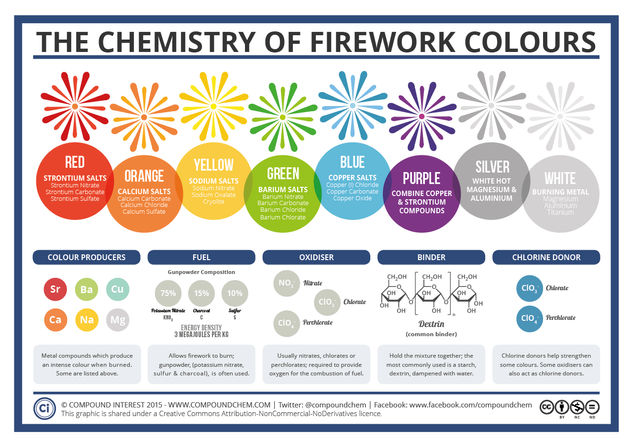
The colours in fireworks stem from a wide variety of metal compounds – particularly metal salts. ‘Salt’ as a word conjures up images of the normal table salt you probably use every day; whilst this is one type of salt (sodium chloride), in chemistry ‘salt’ refers to any compound that contains metal and non-metal atoms ionically bonded together. So, how do these compounds give the huge range of colours, and what else is needed to produce fireworks?
The most important component of a firework is, of course, the gunpowder, or ‘black powder’ as it is also known. It was discovered by chance by Chinese alchemists, who were in actuality more concerned with discovering the elixir of life than blowing things up; they found that a combination of honey, sulfur and saltpetre (potassium nitrate) would suddenly erupt into flame upon heating.